Published on 2025-06-02

Single-use medical devices have become indispensable in an industry where every second counts. These pre-sterilized, ready-to-use tools keep patients safe and maximize efficiency in critical moments.
Table of Contents
What are Single-Use Medical Devices?

Single-use medical devices, or disposable medical devices, are designed to be used once and discarded. They offer a convenient and safe solution for various medical procedures. Standard single-use medical devices include:
- Oxygen masks
- Blood collection tubes
- Syringes and needles
- IV catheters
- Surgical gowns and drapes
Some medical devices, such as forceps and scalpels, are designed for multiple uses. They’re made of durable materials, like stainless steel or titanium, that can be cleaned and sterilized repeatedly.
However, cleaning and sterilizing medical instruments is time-consuming and labor-intensive. If it’s not done correctly, there’s a risk of cross-contamination.
Single-use devices, on the other hand, remove these concerns. Because they are meant to be used and disposed of, they reduce the risk of infection transmitted between patients and improve workflows and resource allocation for healthcare workers.
This is why, in recent years, there has been a surge in demand for disposable medical devices.
Manufacturing Processes for Single-Use Medical Devices
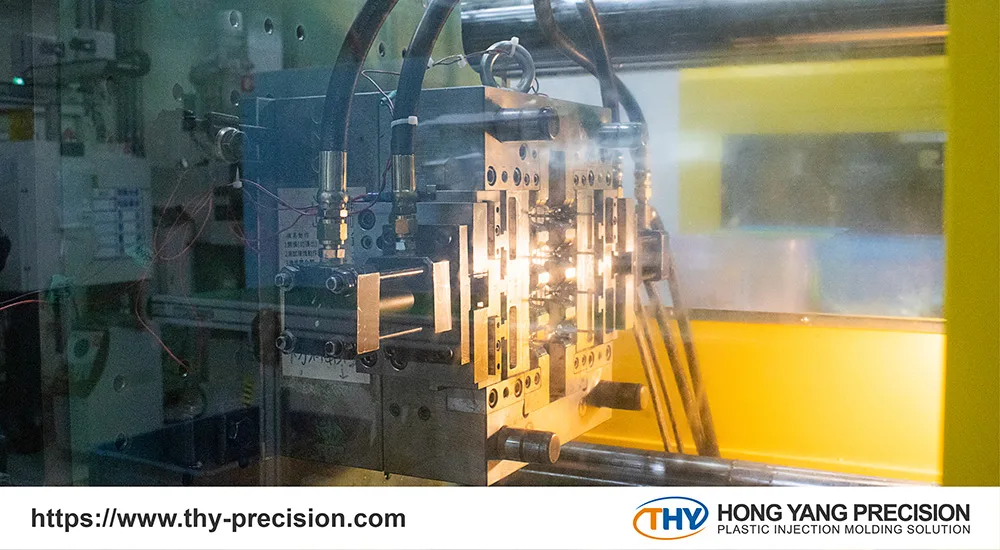
There are four different manufacturing processes for single-use medical devices:
Injection Molding of Plastic Components
Injection molding produces plastic parts with a high level of accuracy and efficiency. Plastic pellets are heated to liquid form and injected into a mold cavity. The liquid cools and solidifies into the desired shape.
Various medical-grade polymers, including polyethylene (PE), polymethyl methacrylate (PMMA), polyvinyl chloride (PVC), and acrylonitrile butadiene styrene (ABS), are utilized in manufacturing. Although the initial mold investment may be expensive, it is cost-effective in high-volume production.
1.Sheet Metal Fabrication and Welding
Many single-use medical devices need custom-made metal components, such as brackets, plates, and adapters, created through sheet metal fabrication and welding.
2.Printed Circuit Board Assemblies (PCBAs)
Printed Circuit Board Assemblies (PCBAs) are the control centers for many medical tasks. The manufacturing process combines electronic parts like sensors, microchips, and memory to create devices that allow medical professionals to automate tasks, monitor data, etc.
They’re typically compact and require special manufacturing techniques to function accurately inside a small device.
3.Human Machine Interfaces (HMIs)
Some single-use medical devices include human-machine interfaces (HMIs), which healthcare workers use to interact with the device — buttons, displays, and touchscreens.
When designing effective HMIs, OEMs must consider factors like ease of use, information clarity, and the device’s purpose.
3 Benefits of Single-Use Medical Devices
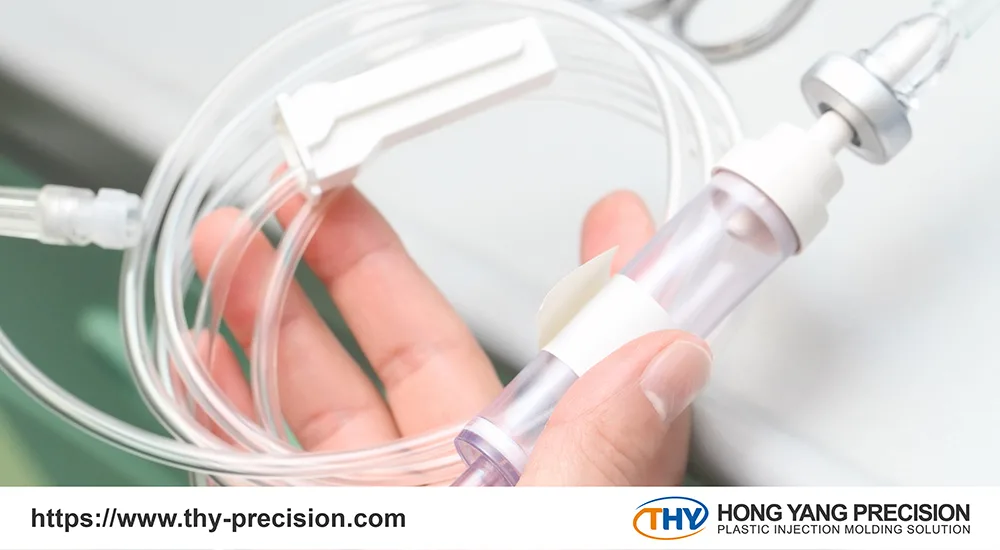
Medical devices have become essential in today’s healthcare environment. As medical technology advances, hospitals and clinics emphasize patient safety, efficiency, and cost-effectiveness.
Single-use medical devices reduce the risk of cross-contamination, help optimize medical resources, and improve overall healthcare quality. Here are three key benefits of single-use medical devices:
1.Patient Safety
Since single-use medical devices are designed to be thrown away after one use, they significantly reduce the risk of cross-contamination and healthcare-associated infections (HAIs). This hugely benefits vulnerable populations and procedures with a high infection risk.
2.Time and Labor Savings
Single-use devices eliminate the time-consuming cleaning, sterilizing, and reassembling of reusable instruments. Thus, healthcare professionals can optimize their time for patient care and other essential tasks.
3.Cost Efficiency
While the initial cost may be higher, single-use devices lead to cost savings in the long run. They reduce the cost of cleaning and reusing medical equipment, which helps lower overall healthcare spending.
3 Potential Risks of Single-Use Medical Devices

Single-use medical devices are meant to be used only once. Reusing them can cause the following problems:
1.Potential for cross-infection
Cross-infection can happen when germs and bacteria are transferred from patient to patient by reusing single-use medical devices. This can lead to serious infections and compromise patient safety.
2.Inability to clean and decontaminate
Although some medical devices are designed for multiple uses and can withstand repeated sterilization, single-use devices are not. They are often made with materials that cannot be adequately cleaned or decontaminated without breaking down or releasing harmful toxins.
3.Residues from chemical decontamination agents
Even with a thorough cleaning, residues from chemical decontamination agents can remain on single-use devices. Patients who use these devices can experience skin irritation, allergic reactions, or other serious complications.
Fortunately, refraining from reusing disposable medical devices significantly minimizes these risks. Dedicated manufacturers specializing in high-quality single-use medical products ensure a safer patient healthcare environment.
These manufacturers provide a wide range of disposable medical devices, such as infusion sets designed for intravenous fluid therapy to maintain sterility and facilitate efficient drug delivery and syringes and needles commonly used for vaccinations, blood collection, and medication administration.
Disposable blood pressure cuffs help prevent bacterial transmission in medical monitoring, and other products further contribute to infection control and patient safety.
Partnering with a Specialized Manufacturer: THY Precision
Precision engineering and quality control are essential for manufacturing single-use medical devices. THY Precision delivers on all fronts.
As an ISO 13485 Certified medical device plastic injection molding manufacturer, THY Precision adheres to the highest international standards for medical device production.
Their commitment to rigorous testing procedures, advanced machinery, and custom design services ensures your single-use devices meet strict quality and safety requirements.
Contact THY Precision today to discuss your next big project.
Learn more:
How to Evaluate and Select a Reliable Medical Device OEM?
Medical Device Implants: What You Should Know
